DTC-0220: Multi Kinase Inhibitor for Ph+ ALL and Ph+ “Like” ALL
Acute Lymphoblastic Leukemia (ALL) is the most common pediatric malignancy and remains one of the leading causes of disease-related deaths in children and young adults. Advances in the treatment of these diseases has led to the identification of subsets that carry poor prognoses. Several of these subsets have been found to have kinase dependent drivers. These subsets include: Philadelphia chromosome positive B-ALL (Ph+ B-ALL), Philadelphia-like B-ALL, and TCF3::HLF B-ALL, as well as T-cell acute lymphoblastic leukemia (T-ALLs). Further studies would suggest an increase in reliance of the Pre-B-cell Receptor in relapsed and steroid resistant B-ALL . As such, these studies would suggest a larger group of patients with ALL that may benefit from targeting of kinases.
Ph+ B-ALL carries the hallmark BCR::ABL1 and confers a poor prognosis with historical outcomes of less than twenty percent. This subset of ALL is seen in 5-25% of ALL with increasing prevalence with age. With the advent of tyrosine kinase inhibitors (TKI’s), outcomes have significantly improved to close to 70% long-term survival in combination with intensive therapies. Unfortunately, relapsed disease remains a significant burden. Of note, patients treated with single agent TKI’s have developed drug resistance, for which the main driver of relapse is T315I mutations in ABL1 in up to 75% of cases. Currently, the only TKI option for these patients is ponatinib (Iclusig™, Takeda). However, ponatinib carries a “BLACK BOX” warning from FDA for “VASCULAR OCCLUSION, HEART FAILURE, and HEPATOTOXICITY”, and is only approved for patients that did not tolerate or no longer benefited from treatment with at least 2 prior kinase inhibitor medicines, or who could not receive any other kinase inhibitor medicines. Recently, interest has reignited for the development of a potentially safer and more effective drug than ponatinib. Early clinical data with ELVN-001 from Enliven Therapeutics and olverembatinib from Ascentage Pharma has generated excitement amongst investors and clinicians.
DTC-0220 is the first “multi kinase inhibitor” which has single nM IC50‘s against ABL1/ABL1-T315I (and most of the clinically relevant mutations of ABL1), BTK, BLK, AurK, JAK2, SRC and LCK. Evidence suggests synergistic anti-cancer activity between ABL1 and AurK and ABL1 and JAK2. Furthermore, there is a known role of BTK in pre-B cell malignancies. Finally, there appears to be a role of LCK in driving some T-ALLs. These facts provide a scientific basis for potentially improved activity of DTC-0220 against CML/ Ph+ ALL and T-ALL.
Philadelphia-like ALL (Ph-like ALL) is another subset of ALL that also confers a poor prognosis. This subset of ALL also has an increased prevalence with age and carries a heterogeneous group of molecular events. Intriguing is that the majority of these subsets fall into ABL class fusions and JAK2 dependency, making DTC-0220 of interest. TCF3::HLF ALL is an extremely rare subset of ALL with an extremely poor prognosis. We recently developed preclinical data suggesting targeting of AURKA activity has potential disease response. Again, DTC-0220 has the ability to target AURKA making this compound of interest for this subset of ALL. Finally, relapsed B-cell ALL has been shown to increase it’s dependence on the Pre-B-cell receptor. Downstream signaling of this pathway include kinases such as BTK. DTC-0220’s ability to also target this kinase may have some potential for therapeutic benefit for this population. As such, DTC-0220’s unique properties of minimal toxicity but significant ability to inhibit multiple kinases makes it an attractive compound to test in these high risk populations of B-cell ALL.
Hypothesis: DTC-0220’s unique properties of inhibition of multiple kinases including ABL1/ABL1T315I, JAK2, AURKA, LCK and BTK has the potential to have therapeutic benefits for high risk subsets of B-cell ALL.
Regulating the Senescence-
Associated Secretory Phenotype (SASP)
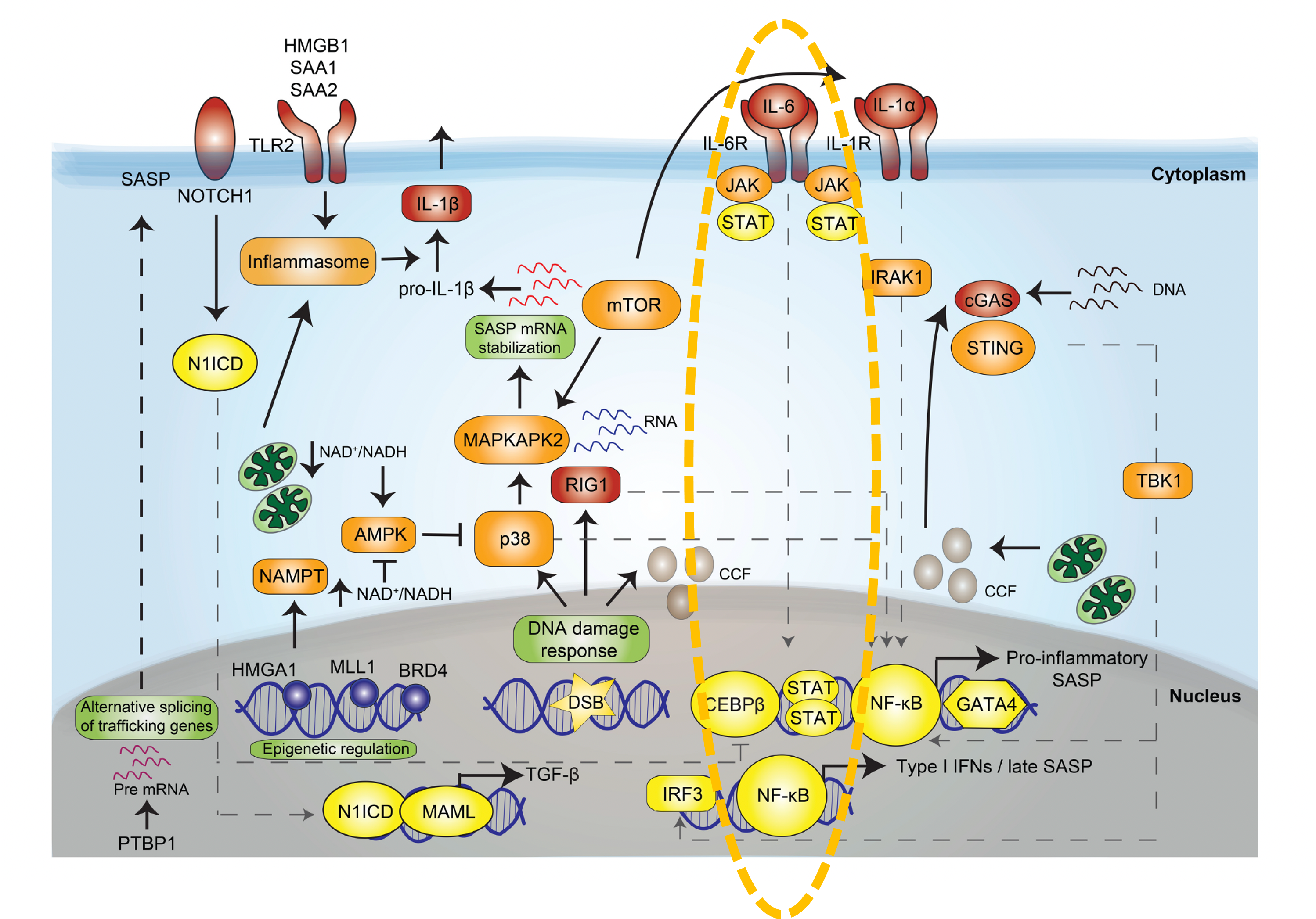
Cellular senescence is characterized by irreversible cell cycle arrest and a proinflammatory senescence-associated secretory phenotype (SASP), which is a major contributor to aging and age-related diseases. Senescent cells accumulate during aging and negatively influence lifespan and promote age-dependent changes in several organs.
IL-1, IL-6, IL-8, TNF-α, TGF-β, and various matrix metalloproteinases (MMP3, 12) make up the key components of the SASP. The sustained inflammatory microenvironment created by the SASP can lead to chronic inflammation, which can have detrimental affects on tissues and organs, contributing to various age-related diseases and conditions. JAK kinases regulate the SASP through the JAK/STAT pathway. We are developing selective JAK2/3 topical TKIs as senomorphic therapy to down-regulate the SASP without the potential negative adverse effects of JAK1 inhibition.